

Due to COVID-19, our annual seminar, VASCERN Days 2020, could not be held in Paris as planned. Luckily, we were able to arrange 3 days of online meetings and networking for our members and invited guests, from October 22-24th, 2020, using Webex for the videoconference calls and Whova as event management platform.
The seminar was attended by 76 Healthcare Provider (HCP) representatives, 12 representatives from our 7 Affiliated Partners and 22 European Patient Advocate Group (ePAG) patient advocates, as well as 35 invited stakeholders representing DG SANTE (European Commission), FAVA-Multi (The French Network for Rare Vascular Diseases), Eurordis, members of our VASCERN Registries Project team, EJP-RD, the French Ministry of Health, AP-HP Bichat Hospital and more!
The first morning started with our Board Meeting, where Professor Guillaume Jondeau (VASCERN Coordinator) and Marine Hurard (VASCERN Project Manager) welcomed all to this special virtual edition of VASCERN Days.
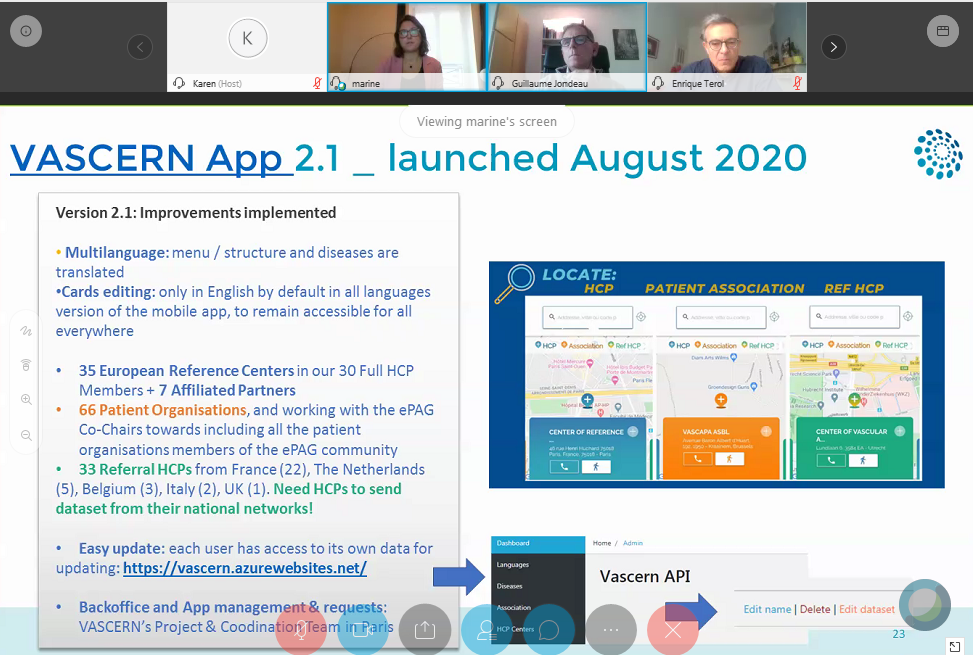
VASCERN’s Management and Coordination, including the monitoring indicators to be collected for semester 1 of 2020, the release of VASCERN App version 2, EU co-funding projects and an update on our communication and dissemination activities, was the first topic of the day. This was followed by VASCERN’s Enlargement & Cooperations, where the 7 new affiliated partners were formally presented and representatives from countries that already have national rare disease networks shared their good practices and advice for other countries looking to set up national networks. Finally a very interesting presentation on the progress of the Inter-ERN Working Group on Legal & Ethical Issues and Relations with Stakeholders by Romain Alderweireldt, our Ethical & Legal Advisory Working Group Chair, was given.
Next up was the discussion on Work Packages and their Implementation in VASCERN’s 5 Rare Disease Working Groups (RDWGs) with Professor Claire Shovlin (HHT-WG Chair), Professor Julie De Backer (HTAD-WG Chair), Doctor Leema Robert (MSA-WG Chair), Doctor Robert Damstra (PPL-WG Chair) and Professor Miikka Vikkula (VASCA-WG Chair) giving updates on what their groups had accomplished in the last year.
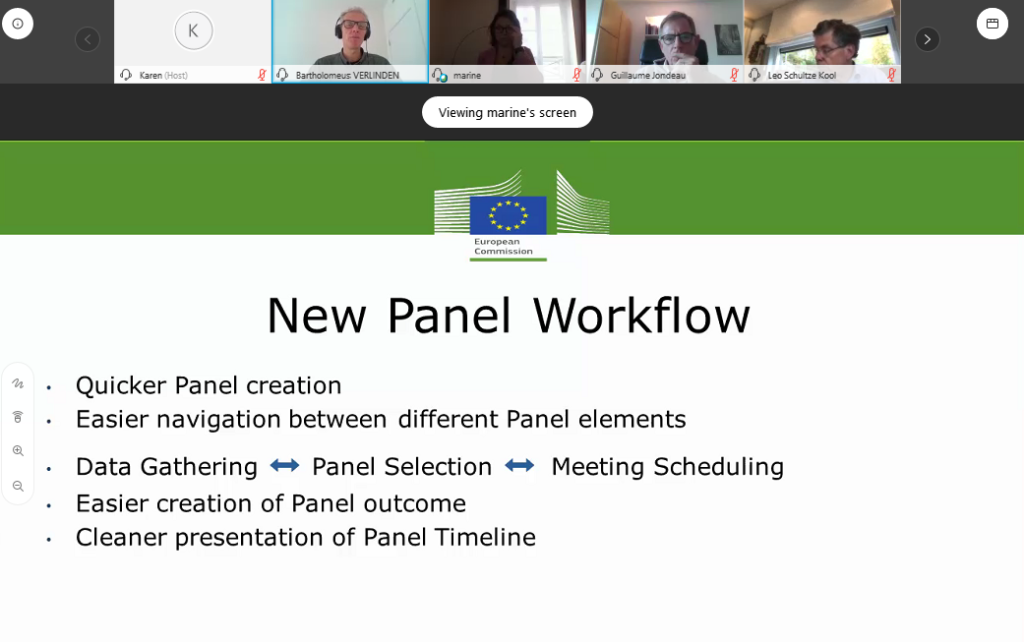
After a brief question and answer session, where we were grateful for the participation of Enrique Terol (Policy officer, DG Sante), the terms of reference were approved by the board. The plenary session continued with a presentation by the European Patient Advocacy Group (ePAG) on their views, projects & new developments and finally a presentation on the Clinical Patient Management System (CPMS) by Bart Verlinden (ERN Project Manager Information Systems Unit – External Consultant at DG Sante).
In the afternoon, the RDWGs held parallel sessions in order to progress on their various work packages. These sessions concluded with the HCP representatives from each group taking time to discuss several complex patient cases using the CPMS, with HTAD and MSA members coming together for joint discussions.
A brief council meeting was held at the end of the day in order to select 5 topics for the following day’s brainstorming session. The topics selected were those chosen by each of the 5 RDWGs and were: accessibility to genetic testing (HHT), how to reach uniformity of treatment (medical/surgical) in Europe (HTAD), how to encourage more centers in larger countries to apply and how to favour creation of new centers in smaller countries (MSA), psychology in rare diseases (PPL) and national networks (VASCA).
Day 2 started with a presentation on the European Joint Programme on Rare Diseases (EJP RD), by Daria Julkowska and Yanis Mimouni, which was of great interest to our members as they outlined all of the current and upcoming funding opportunities as well as support offered to the ERNs. This was followed by a presentation on VASCERN’s Registries Project by Professor Leo Schultze Kool, Chair of VASCERN’s Registry WG, with the latest updates and progress.
Next was the lively brainstorming session with the 5 topics decided by the board the day before. See the topics and ideas that were discussed here.
During the lunch break there was also the first meeting of VASCERN’s Pregnancy Working Group, chaired by Professor Julie De Backer.
The RDWG sessions were held for the rest of the afternoon (including MSA&HTAD and VASCA&PPL interRDWGs sessions) until everyone convened again for a final summary session on each RDWG’s meeting outcomes and what they have planned next:
- HHT: The HHT-WG reported very productive and enjoyable sessions. After settling on the topic for the brainstorming session on the first day, they introduced 5 additional topics that each HCP gave their own experience on, leading to interesting discussions. They will be generating a survey on genetic accessibility, as discussed in the brainstorming session. They also had 3 case discussions, 2 of which involved obstetric cases where 2 resident obstetricians from London and Lyon were able to join the group and provide their expertise. They created 2 new Do’s and Don’ts statements on screening and treatment of gastrointestinal bleeding in HHT. They also agreed that there is a need for a statement on nosebleed management. The group reviewed the use of telemedicine across the HHT-WG’s HCPs, discussing what works well and what financial and legal aspects to consider. They also decided that they would like to develop webinars for patients, where questions can sent in advance for clinicians to answer, as well as the possibility of live translations in order to make webinars more accessible to all.
- HTAD: The HTAD-WG had a productive meeting with the MSA-WG with a discussion on their patient pathways and the evaluation of their pathway. Clinical outcome measures were also discussed and, with close collaboration of the HTAD patient advocates, a survey will be sent to healthcare providers and patients in order to advance on this topic. The HTAD registry was discussed, as was another project with the aim of having a streamlined way of measuring the aorta on scans and a strategy on doing this using the CPMS is being set up. There was a discussion on creating a compendium of definitions and methods for the assessment of the clinical manifestations of HTAD that would be useful to care providers and patients. They continue to advance in their production of new Pills of Knowledge (PoKs). Finally, they enjoyed their patient case discussions, where they discussed 7 cases using the CPMS.
- MSA: The MSA-WG discussed the importance of the integration of their two new diseases, Fibromuscular dysplasia (FMD) and Spontaneous Coronary Artery Dissection (SCAD) as well as how they will handle the issue of Brexit within their group and its impact. They had several patient case discussions involving vascular Ehlers-Danlos (vEDS). They reported how they have produced many webinars and PoKs this year. They also let everyone know that their focus for the next year will be on producing consensus documents and updating their patient pathway and Do’s and Don’ts documents.
- PPL: The PPL-WG started with a discussion of the FAIR registry, including their specific PPL data elements and how to proceed. They created several sub-working groups (on obesity, severity scoring lists, and psychology) that they feel are needed. They will soon expand their Do’s and Don’ts with a new chapter on skincare, and they will shortly be releasing a new PoK on adolescence and lymphedema (created by Italian PO and validated by the group). Several other PoKs are also being filmed by the PPL patient advocates. They will work on a new publication, that will involve a retrospective collection of pediatric lymphedema patient data from 2019. Their highlight was the patient case discussions, of which they had 11 (some with the VASCA-WG)!
- VASCA: The VASCA-WG, who held an additional session on Saturday October 24th, 2020, were very productive during their three sessions. Two PoKs, created by the patient organisation HEVAS were validated by the group, and will be released shortly. They equally advanced on their plans to make PoKs that will explain their patient pathways and came up with a list of topics for future position statements. The important topic of national networks was also explored, with the group brainstorming ways they could help countries in creating national networks where there are none, such as collecting information in each country on possible expert centers and providing the criteria for VASCA expert centers. The VASCA registry was discussed, including the specific items that need to be added for specific conditions. The VASCA patient advocates also presented their project of a VASCA magazine, which was approved by the group.
Our coordinator, Professor Guillaume Jondeau, then closed the meeting by congratulating everyone on all the work accomplished. Despite our annual seminar being held online, and not meeting face-to-face, we were still able to work efficiently and make lots of progress!
On Saturday, the VASCA-WG held one last meeting where they went over all of the sub-working group projects underway and what next steps need to be taken. The European Patient Advocacy group (ePAG) also held their official meeting, with Anne-Laure Aslanian (Patient Engagement Manager Healthcare – ERN & Healthcare / European Patient Advocacy Groups, Eurordis), where they provided their feedback and ideas for improving the VASCERN website and many other important topics.
We thank all participants for making VASCERN Days 2020 such a success! Our members have lots of exciting projects lined up and we look forward to sharing updates on them soon.
See full agenda here
Presentation slides for the plenary sessions can be consulted below:
Day 1 VASCERN Days 2020 Board session (October 22nd)
Day 2 VASCERN Days 2020 Plenary session (October 23rd)

