
On the 6th and 7th of October, 2022, VASCERN held its annual seminar in Paris, France. It was a great pleasure to welcome our members and invited stakeholders from EJP-RD, EURORDIS, Fondation AP-HP, AP-HP Hôpital Bichat, Orphanet, members of our VASCERN Registries Project team, and FAVA-Multi (The French Network for Rare Vascular Diseases) to VASCERN Days 2022 once again in Paris and without the masks from last year. We also provided a hybrid option so participants who could not make it to Paris could still connect and join us virtually. At this seminar, participants discussed various upcoming projects such as webinars on pregnancy in Lymphedema, patient pathways for CADASIL, integration of Microsoft Teams, and possible developing partners. Have a read through this article to know more about all the activities of these two days!
As in previous years, the seminar kicked off with the annual Board meeting, where VASCERN Coordinator Professor Guillaume Jondeau welcomed everyone and introduced the VASCERN Project Manager, Julie Hallac; VASCERN Communication and Patient Engagement Officer, Treasure Udechukwu; VASCERN Administrative Project Officer, Yael Glin; VASCERN Science & Education Officer, Gloria Somalo; VASCERN IT Helpdesk & End-User Support Specialist, Ibrahim Donmez; and VASCERN Data Steward, Pim Kamerling. Following that, Julie Hallac provided updates on the EU co-funding projects for VASCERN, and Treasure Udechukwu provided information on VASCERN’s communication efforts (including new communication strategies). Gloria Somalo and Yael Glin also presented on current training and educational activities, such as the ERN exchange programme.
ePAG advocate and Chair of the VASCERN Ethics Working Group, Romain Alderweireldt, discussed the most recent developments from the Legal & Ethical Issues and Relations with Stakeholders Working Group (LES-WG), including a partnership with Together for Rare Diseases (Together4RD) to support ERNs in working with relevant stakeholders to solve the unmet medical needs of patients with rare diseases. He also spoke about the 101 Genome project, which aims to identify modifier genes within the whole human genome that can explain the variability of cardiovascular disease found in people with Marfan syndrome. Ibrahim Donmez also presented on IT developments and the integration of Microsoft Teams. Finally, there were updates on the ERN evaluation and VASCERN expansion.

The latest news from the transversal Working Groups (Pregnancy and Registries) were shared. European Patient Advocacy Group (ePAG) Co-chair for Pediatric and Primary Lymphedema Working Group (PPL-WG), Pernille Henriksen and Charissa Frank, ePAG Co-Chair for Medium-Sized Arteries Working Group (MSA-WG) made a presentation on the growth of the ePAG including welcoming the new ePAG advocates from the Neurovascular Diseases Working Group (NEUROVASC-WG), their representation and work in VASCERN, and news on the ePAG Patient Partnership Working Group.

After a brief coffee break, we were joined by Daria Julkowska from EJP-RD, who presented on the many research funding available, and updates on the European Partnership on Rare Diseases, which is expected to launch in 2024. The six chairs of the Rare Disease Working Group (RDWG), Prof. Sophie Dupuis-Girod (Hereditary Hemorrhagic Telangiectasia Working Group (HHT-WG)), Prof. Julie De Backer (Heritable Thoracic Aortic Disease Working Group (HTAD-WG), Dr. Michael Frank (Medium-Sized Arteries Working Group (MSA-WG)), Dr. Dominique Herve (Neurovascular Diseases Working Group (NEUROVASC-WG))Dr. Robert Damstra (Pediatric and Primary Lymphedema Working Group (PPL-WG)), and Prof. Miikka Vikkula (Vascular Anomalies Working Group (VASCA-WG)) provided updates on the implementation of the VASCERN work packages. Following an engaging round of questions and answers, the Board eagerly approved all of the projects that were presented.


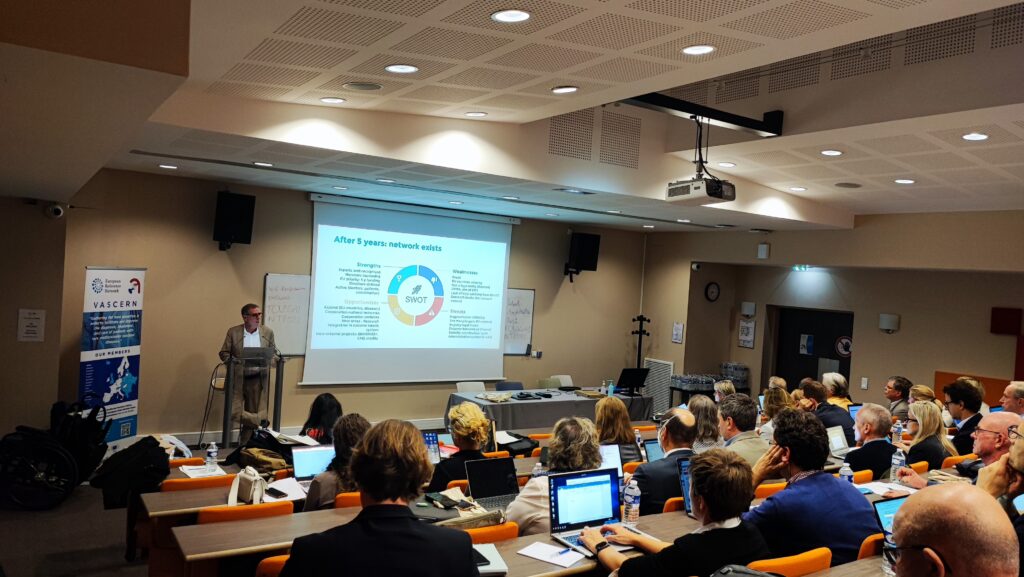
For the second half of the plenary session, Pim Kamerling gave a presentation on data quality and how crucial it is for registries. The group was then divided into their respective transversal parallel sessions (Pregnancy and Registries), as well as a brainstorming session. The brainstorming session focused on the strengths, weaknesses, threats and opportunities of the network in the last 5 years. Healthcare professionals and patient advocates had the chance to express their opinions and engage in an open and honest discussion. There were extensive discussions about ways to grow the network, raise awareness of VASCERN’s resources, and work with other national networks and scientific societies. It was also proposed that other healthcare professionals involved in the treatment of patients with rare diseases, such as nurses and psychologists, be included in meetings and case discussions.

For the rest of the afternoon, the group split up into their six RDWGs for their first rare disease working group parallel sessions. Later that evening, attendees were welcomed on a boat for a networking dinner, where they exchanged many ideas, memories, laughter, and glasses of wine!




On the second day of the seminar, Lenja Wiehe and Rita Francisco from EURORDIS joined the patient advocates at the VASCERN ePAG meeting while the healthcare provider representatives of each RDWG had Clinical Patient Management System (CPMS) case discussions. The rest of the day was devoted to the second and third round of parallel RDWG sessions to wrap up specific projects and make future plans. The HHT, VASCA, and NEUROVASC WGs, PPL and VASCA WGs, and the MSA and HTAD WGs also met jointly for a session to discuss their shared topics and challenges.

Each of the RDWGs made significant progress on numerous projects as a result of the three RDWG sessions over the course of two days. Here are some key conclusions from the groups:
Hereditary Hemorrhagic Telangiectasia Working Group (HHT-WG)

- They finished their contraception questionnaire and agreed on the next steps.
- Creation of a Do’s and Don’ts fact sheets on blood transfusion and organ donation was agreed on.
- It was decided to hold a webinar on pregnancy in HHT early next year.
- Discussed the criteria and collaborations with a national expert centers to find new members for the network.
- 3 complex clinical cases were discussed using the CPMS.
Heritable Thoracic Aortic Disease Working Group (HTAD-WG)

- The status of the HTAD Registry was discussed, and the data elements were described in more detail.
- The group reviewed the many papers published by HTAD-WG members and discussed on the findings.
- Updates on the aortic measurement project were discussed including the standardization of the aortic output measurement.
- It was decided that the outcome measures in HTAD will be published as a recommendations for physicians.
Medium-Sized Arteries Working Group (MSA-WG)

- There was discussion regarding the questions that were sent for their upcoming webinar on ‘Everything you wanted to know about vEDS’.
- The Delphi questionnaire was finalized, and it was decided which invited experts would participate.
- The e-learning module for the MSA-WG was discussed, and next steps were decided.
- The registry data elements were reviewed and adapted for several EU countries.
NEUROVASC WG
- The CADASIL and Moya Moya registries’ variable lists were validated.
- New projects such as the Do’s and Don’ts factsheet for both CADASIL and Moya Moya, were given to various members of the group.
- Collaborations with VASCA-WG for clinical case discussions requiring the expertise of a Neurosurgeon or Neurologist were discussed.
- The application for EJP-RD financial grants was discussed.
Pediatric and Primary Lymphedema Working Group (PPL-WG)

- Addition of 3 new Orphanet codes were discussed with an Orphanet representative and the process will begin soon.
- A new pregnancy PoK in German with FAQ in multiple languages was decided on.
- Collation of information and questions to be answered in a webinar on genetics were discussed.
- It was agreed to make a PoK on tattoo for lymphedema patients.
- The group discussed about writing a paper on the Pediatric and Primary Lymphedema patient pathway.
- 7 complex clinical cases were discussed in the CPMS.
Vascular Anomalies (VASCA-WG)

- The decision was made to apply for the EJP-RD networking grant.
- There were extensive discussions about working with national networks on research projects.
- The group discussed and divided up the topics for the second issue of the VASCA Magazine.
- The group went over the first draft of the AVM diagnosis and management pathway.
- The paper on the capillary malformation pathway has been completed and will be submitted soon.
- 4 complex clinical cases were discussed in the CPMS.
The seminar concluded with a brief closing session where Professor Jondeau thanked everyone for coming and wished them safe travels home.

We sincerely appreciate everyone who participated in this edition of VASCERN Days, both in person and online. We look forward to the next VASCERN Days!

